Tourmaline Queen Mine Pala, San Diego County, California Caltech Collection | Blue Cap Tourmaline From the 1971-1972 find at the Tourmaline Queen Mine Light & Stone Collection: Photo Credit: Harold and Erica Van Pelt |  Tourmaline King Mine Pala, San Diego County, California Caltech Collection |
What is a blue cap tourmaline?
A blue cap tourmaline is a bicolored tourmaline with a pink body and a blue colored tip at the termination end.From where do they come?
In the USA they are found near Pala, San Diego County, California, at the Tourmaline Queen Mine where a major find occured in 1972, and at the Tourmaline King Mine.Brazil has several mines. In particular, they have come from the Sapo Mine, Hinga, Minas Gerais, Brazil.
The Sapo mine produced many blue caps and many of these were “shiny”.
What is the origin of the blue color in the cap?
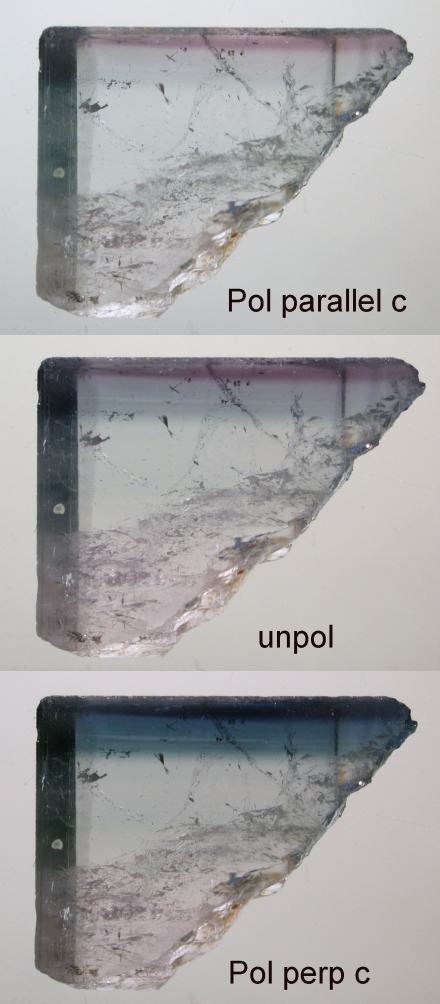
To explore this, we will examine a blue cap crystal from the Tourmaline Queen Mine, Pala, California.In what follows, you will see images take in both polarized and unpolarized light.
Next, we look at the optical absorption spectra of the different colored zones in the slice of the blue cap tourmaline
Zones used for Spectral Studies
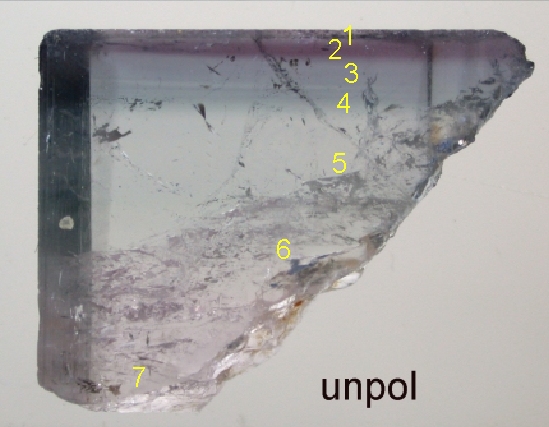
Approximate location of the different color zones used for spectroscopic studies
1) pink with blue at cap
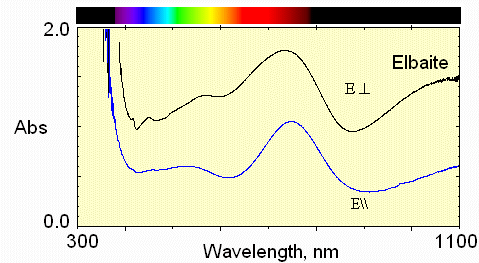
This spectrum shows Fe2+ absorption at about 1100 nm and 735 nm.
Mn3+ absorption occurs near 530 nm. Fe2+ - Ti4+ interactions are weak at 430 nm.
In the E\\c polarization, the greatest transmission (least absorption) in the visible region is in the 620 nm region, namely reddish.
2) purely blue zone near cap
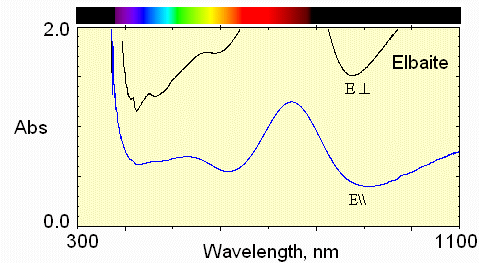
The iron absorption is stronger here, but the Fe-Ti interaction remains weak.
Now, in the E perpendicular to c polarization, the greatest transmission is in the blue region.
3) dark blue zone
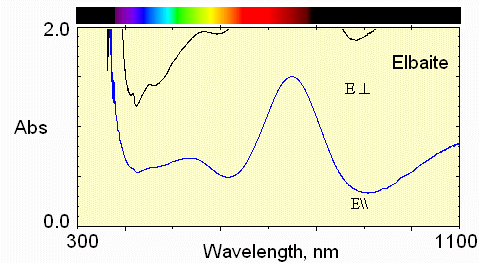
Still stronger absorption by Fe2+ and only transmission in the blue region for light polarized perpendicular to the c-axis
4) paler blue region
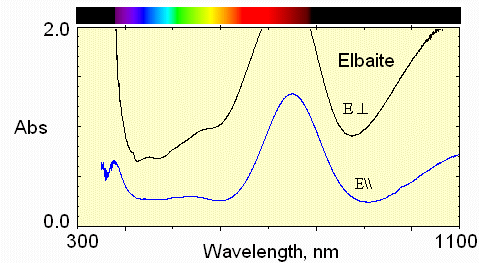
Less Fe and less Mn absorption.
5) Pale above crack
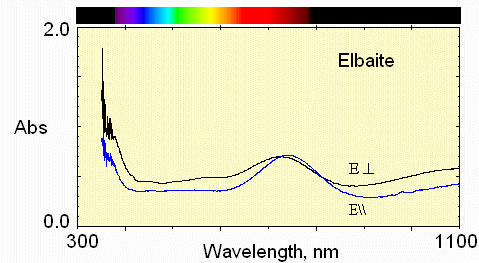
6) Pale below crack
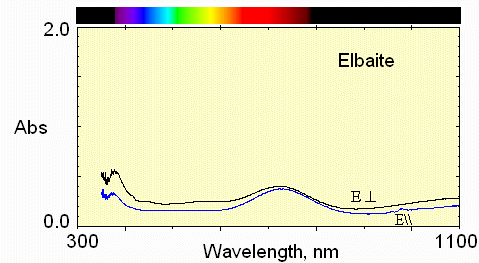
Much less iron absorption and little manganese.
7) Bottom region
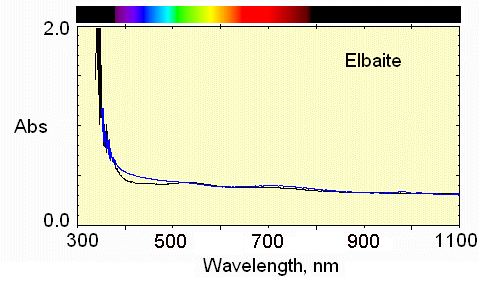
Very pale color, but the weak Mn3+ absorption near 530 nm causes a pale pink in the E perpendicular to c direction.
What makes the blue in the blue cap.
1) Absorption by Fe2+
Iron removes the red portion of the spectrum which allows the blue color to dominate.
2) Lack of significant amounts of Ti4+
If Ti were present, it would interact with Fe2+ to give intervalence charge transfer and make the cap green.
3) Low concentration of Fe3+
If there were a lot of oxidized iron (Fe3+), it would interact with the reduced iron (Fe2+) to cause very intense colors that would probably look black in the thickness of the crystal.
A low concentration of oxidized iron causes a mild Fe2+ - Fe3+ interaction that causes a tiny bit more color in the E perpendicular to c direction than in the E parallel to c direction.