 |
 |
Even within the group of Fe-containing tourmalines, the amount and oxidation
state of the iron can be an important factor in the color of the mineral.
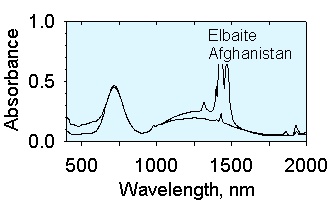
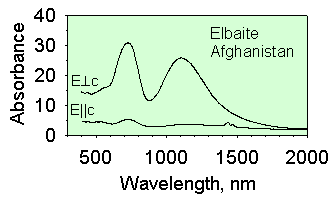
The spectra below are from two elbaites with different iron concentrations.
The one on the left has mostly Fe2+ at low concentration whereas
the one on the right has both Fe2+
and Fe3+ at higher concentrations. At high concentrations
Fe and Fe interact with each other to cause strong absorption when the
incoming light is linerally polarized in the direction perpendicular to
the c-axis (E^c).
 |
 |
Back to the Spectra page
Back to the Mineralogy page