The temperature dependence of hydrous components in minerals
A summer undergraduate research project of
Paul Magyar
OH and water molecules in the nominally anhydrous minerals can be
readily detected by infrared spectroscopic methods. One of the
challenges is determining when water is present as fluid inclusions
rather than as bound molecules in the coordination sphere of cations.
Two of the criteria we use are the temperature depencence of the
IR or NMR spectrum as a function of temperature. Water in liquid
inclusions will usually freeze at or slightly below 0°C. When it
does, the change in the infrared spectrum is particularly obvious.
The change is not obvious, however, in a set of minerals that have
nano-inclusions (dimensions on the order of a few 10's of nm). These do
not freeze in the fashion of larger inclusions and are furthermore
difficult to identify because they are so small that they are
essentially invisible under the optical microscope.
To characterize water in both nano- and micro-inclusions we are
examining the temperature depencence of water in selected minerals that
are known from scanning electron microscope studies to have small
inclusions.
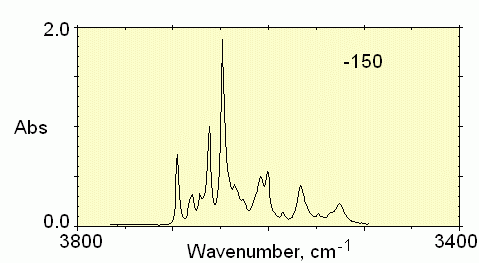
Figure 1, below, shows the typical temperature depencence of the OH
bands in a minerals with only bound OH ions. The mineral is grossular
garnet from East Africa.
Click on the image to animate the spectrum as a function of temperature.