Some gem-quality
labradorite phenocrysts in Miocene basaltic lava from Lake County,
Oregon, have a pink schiller due to metallic copper; some have a
transparent red or green color. The copper content of the crystals
vsries systematically with color: pale-yellow lalradorite sections have
0-40 ppm CuO; greens have about 100 ppm CuO; reds have 150 to 200 ppm
CuO; schiller-bearing laths have 80 to 300 ppm CuO. The variation of Cu
content among different crystals is primary and reflectss a variation
in magma chemisty during plagioclase fractionation. Similarity of
absorption spectra of the red zones to that of copper-ruby color in
glass shows that the red arises from the intrinsic absorption of
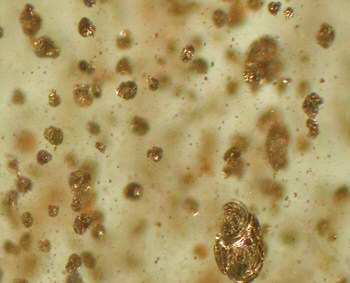
colloidal Cu
0 particles that are too small to
scatter light (<22 nm). Particle size depends on Cu content
because the temperature at which copper begins to exsolve from feldspar
increases with Cu content and the higher temperatures promote
diffusion. At 900 to 1100 °C the reduction of Cu is controlled by
reactions in the basalt that keep
fO2
near the QFM buffer. The green color may be caused by either Cu
1+/Cu
0
IVCT or Cu
0 pairs.
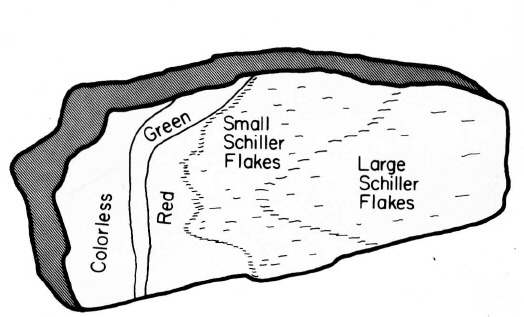
Different zones of color in Lake County labradorite.