Crystal Chemistry of Aquamarine from the True Blue
Showing, Yukon Territory
Lee A. Groat
Department of
Earth and Ocean Sciences, University of
British Columbia, Vancouver, British Columbia V6T 1Z4
George R.
Rossman
Division of
Geological and Planetary Sciences, California Institute of
Technology, Pasadena, California 91125-2500, U.S.A.
M. Darby Dyar
Department of
Astronomy, Mount Holyoke College, 50 College Street,
South Hadley, Massachusetts 01075, U.S.A.
David Turner
Department of
Earth and Ocean Sciences, University of British Columbia,
Vancouver, British Columbia V6T 1Z4
Paula M. B.
Piccoli, Arthur J. Schultz
Intense Pulsed Neutron Source, Building 360, Argonne
National Laboratory, Argonne, Illinois 60439-4814, U.S.A.
Luisa Ottolini
CNR-Istituto di Geoscienze e Georisorse (IGG), Sezione di Pavia, Via Ferrata 1, I-27100 Pavia, Italy
ABSTRACT
Dark blue aquamarine and beryl were
discovered at the True Blue showing in the southern Yukon Territory in 2003. Electron
microprobe compositions show up to 5.39 wt.% FeO in the darkest material, which
is among the highest Fe concentration known for true beryl. Al
site totals average 2.05, with a maximum of 2.10 apfu, which implies that there is more Fe present in the sample
than can be accommodated at the Al
position. Charge-balance considerations
and Mössbauer spectra show that the Fe is present as both Fe2+ and
Fe3+. Optical absorption and
Mössbauer spectra and the results of the X-ray and neutron single-crystal
refinements suggest that there is no Fe at the tetrahedral or channel
sites. Previous studies have proposed
that the color of blue beryl is due to intervalence
charge-transfer (IVCT) between Fe2+ and Fe3+
cations. The anisotropy of the optical
absorption spectra suggest that that if the mechanism responsible for the color
in our samples is IVCT, the vector between the ions involved must be oriented
approximately parallel to c. The only vectors that fulfill this condition
and have a realistic length (2.300 Å) are 4d–Al and 6g–Be. Given the close proximity of the Si positions (closer than any anion
sites), it is difficult to conceive of substitution taking place at the
interstitial 4d site. However Fe could substitute at the interstitial
6g position, but likely only in very
small amounts, because of the need to maintain local charge balance. Unfortunately there is no evidence of this in
the Mössbauer spectra or in difference Fourier maps of the X-ray and neutron
diffraction data. For the former
technique, it is likely that any doublet arising from Fe in the 6gO6 polyhedron is too similar
to the Fe in the AlO6
octahedra to be resolved for either Fe2+ or Fe3+. Calculations suggest that the concentration
of Fe involved in the IVCT process is 0.08 apfu
Fe, of which half (0.04 apfu, 0.17
e-) would potentially be at the interstitial site. This amount of electron and nuclear density is
likely too small to be seen on the difference Fourier maps.
Dark blue aqumarine from the Tatu Mine, Minas Gerais Brazil, is one of the darkest blue beryls discovered, to date.

True Blue beryl on matrix
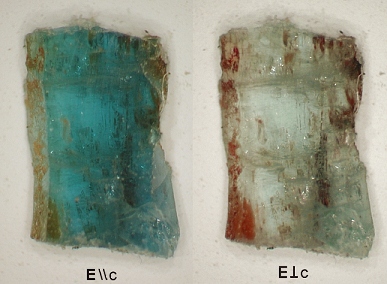
Polarization anisotropy of the True Blue beryl
Additional images of True Blue beryl in matrix
Additional closer view of the locality
Canadian Mineralogist 48, 597-613

