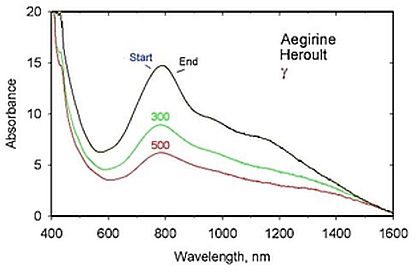
from room temperature to 500 °C
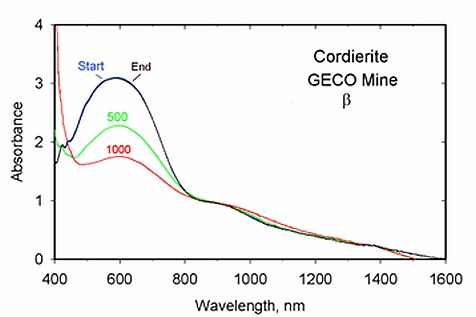
GRR262 cordierite. β orientation spectra
from room temperature to 1000 °C
The absorption
of light by Fe/Ti and Fe/Fe intervalence charge transfer (IVCT) bands
has previously been found in alumnium oxide and Al2SiO5
aluminosilicate minerals to decrease markedly at elevated temperatures.
A broad survey of the optical absorption spectra at elevated
temperatures of various mixed valence iron minerals has been conducted.
The systems considered here are cordierite, chloritoid, lazulite,
dumortierite, jeremejevite, beryl, osumilite, biotite (mica), pargasite
(amphibole) and aegirine (pyroxene). All samples lose significant Fe/Fe
IVCT feature intensity at temperature. In beryl, osumilite, biotite,
pargasite and aegirine, spin-allowed Fe2+ d-d features also
decrease in intensity at temperature; in all but beryl, the intensity
loss is significant. This trend is consistent with d-d band enhancement
via Fe2+/Fe3+ exchange coupling, which has not
previously been identified in the majority of these systems. It is
contrasted against the behavior of ordinary spin-allowed Fe2+
d-d bands in non-IVCT minerals forsterite (olivine) and elbaite
(tourmaline). The depletion of Fe/Fe IVCT and enhanced Fe2+
d-d band intensity may both be important mechanisms by which
iron-bearing mineral phases become more optically transparent under
conditions at depth, which has potential implications for mantle
geophysical processes such as radiative conductivity.

