Spectroscopic probes ranging in energy from gamma rays to
microwaves play an important role in contemporary mineral
studies.
Visible spectra are used to identify the cations which are present in a mineral, their concentration, their crystallographic site, and the identity of ions in the immediate vicinity of the target ion. Metal ions such as V3+, Cr3+, Mn2+, Mn3+, Fe2+, Fe3+, Ni2+, Cu2+ , and UO22+ either alone, or in combination, are responsible for the colors of most common silicate, sulfate and phosphate minerals.
An example of an application involves the identification of the origin of color of two blue tourmalines. Although their color is similar, one contains copper and the other contains iron. The optical spectra show conclusively the origin of the color.
The pyrope-almandine garnet series provides examples of some of the systematics encountered in the spectra of minerals. In these garnets, Fe2+ occupies the large, eight-coordinated site. The band positions in the optical spectrum of the garnet vary with the Fe/(Fe + Mg) ratio and the intensity of the bands is proportional to the total Fe2+ content.
Our students and postdocs have played a prominent role in the
development and application of these methods. Many examples of
visible spectra are to be found on our Mineral Spectroscopy
Server
Special attention is directed at systems where the spectroscopic response is not proportional to the sum of the response of the individual components. Intervalance charge transfer processes which involve two or more cations which can exist in different oxidation states are most important. The Fe2+ - Fe3+ and Fe2+ - Ti4+ interactions, which are particularly important in establishing the color of many common minerals such as micas, amphiboles, and pyroxenes, have been the object of much research.
The interaction between Fe2+ and Fe3+ can also change the intensity of the Fe2+ bands in host minerals such as tourmaline. This will cause the color of the two crystals to be different.
Interactions between pairs or clusters of the same ion are also prominent in mineral spectrocopy. The most important in the mineral sciences is the interaction between pairs of Fe3+. The intense red color of many soils is due to interactions among Fe3+ ions in hematite. Many other Fe3+ minerals such as ferric sulphates and phosphates have appropriate crystal structures to favor interactions between adjacent cations. The result is enhanced intensity and antiferromagnetic interactions.
Infrared spectra can be used to identify minerals, to analyze components in the minerals, to establish the orientation of components in the structure, and to detect phase changes.

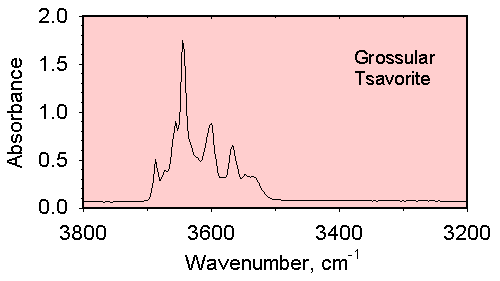
Infrared spectrum of a grossular garnet in the OH stretching region which shows multiple absorption bands which indicate that OH ions occur in a variety of structural environments in this crystal.
In the infrared region of the spectrum, the motions of OH, CH and NH bonds are readily detected.
For many years, we have focused on the analytical calibration of infrared spectra for the determination of the content of hydrous components (water molecules and OH- ions) in minerals nominally considered to be anhydrous. These minerals constitute a major reservoir of water in the earth, probably exceeding the amount of water in all the oceans today.
Raman spectra have become an important means of identifying minerals and synthetic products.